Label-free Imaging Cytometry
Label-free imaging cytometry for live cell population studies by tracking and quantifying individual cells over time — a technical explanation.

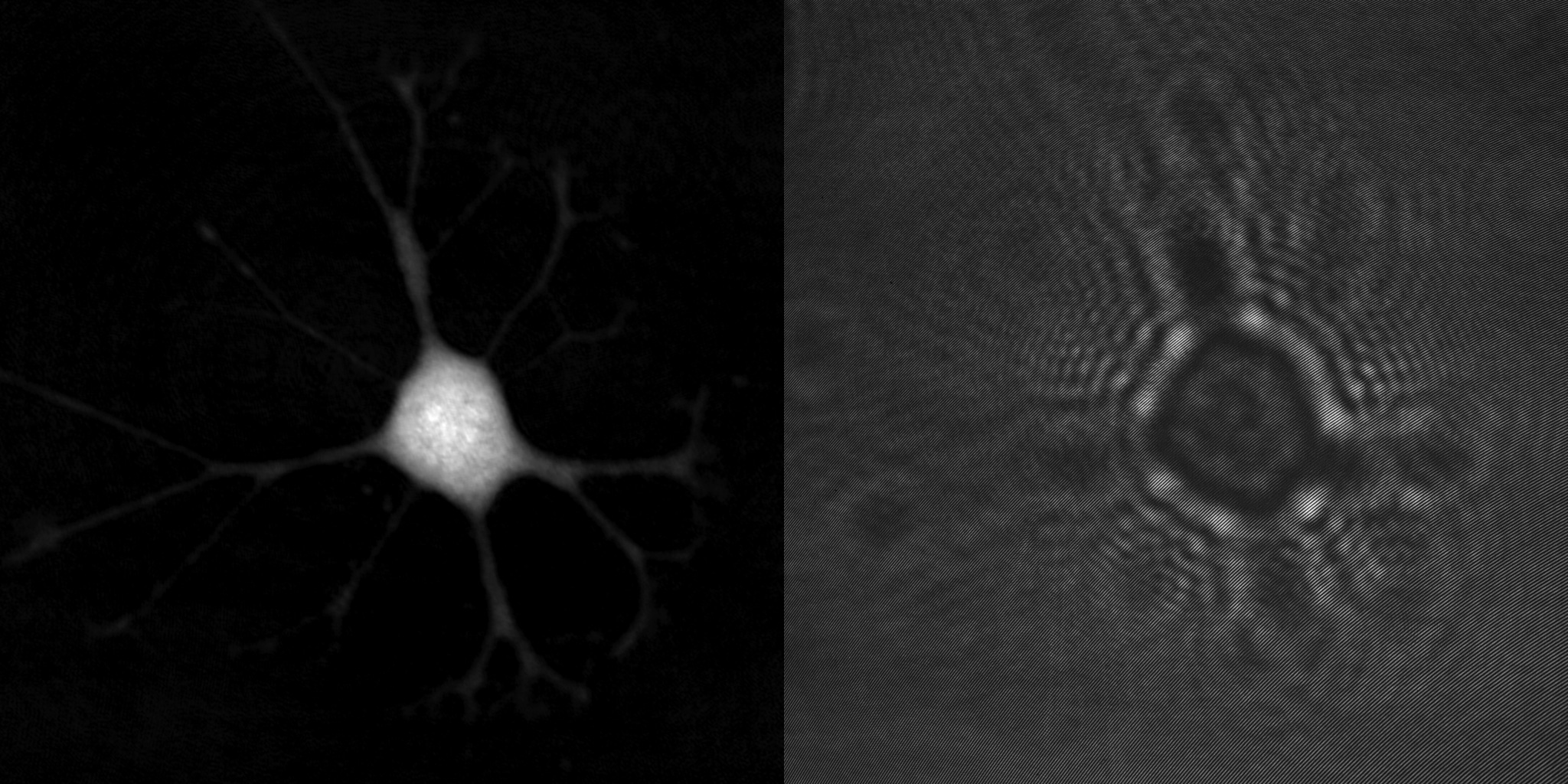
The HoloMonitor® label-free live cell imaging cytometer is based on the principle of quantitative phase imaging, enabling non-invasive visualization and quantification of living cells without compromising cell integrity.
Here we describe the rationale and advantages of using quantitative phase imaging for live cell kinetic analysis of cellular events — explaining the power of live cell time-lapse imaging cytometry and how cells are made visible without labels or stains.
Label-free Imaging Cytometry Saves Precious Cells
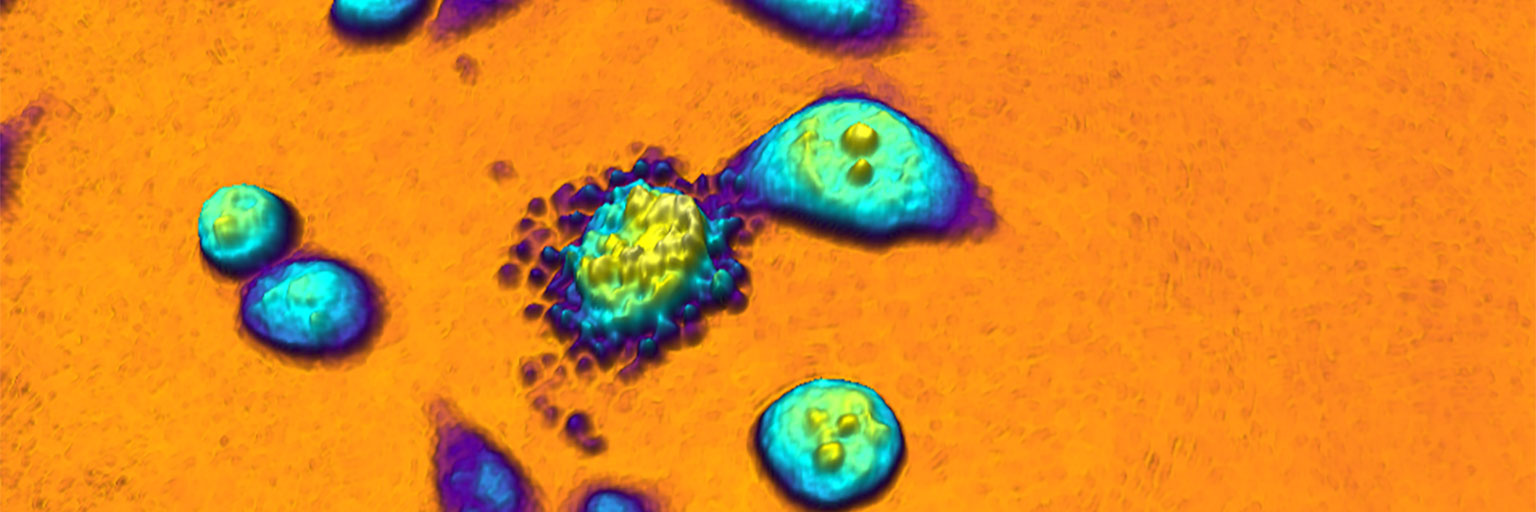
Conceptually, the HoloMonitor cell imaging technology operate differently than conventional cytometric assays. When using traditional assays, the cells are specifically prepared according to the selected assay and can seldom be used for other assays.
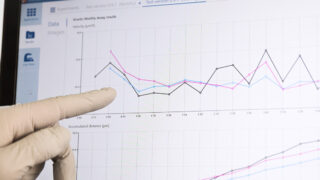
When working with HoloMonitor, the live cells are generically prepared and imaged. After recording, one or more HoloMonitor assays are applied on the time-lapse image sequence to obtain multiple results from the same cell sample, as illustrated below.
Novel Approach
This novel cytometric approach reduces the necessary amount of lab work. But, more importantly, it facilitates the use of precious primary cells over cell lines to improve clinical relevance by dramatically reducing the number of cells needed to obtain results.
Sebesta et al., HoloMonitor M4: holographic imaging cytometer for real-time kinetic label-free live-cell analysis of adherent cells, Proc. SPIE BiOS (2016)
“The HoloMonitor® label-free live cell imaging system is based on the principle of quantitative phase imaging, enabling non-invasive visualization and quantification of living cells without compromising cell integrity.”